Digitize Without Disrupting Compliance
Pharmaceutical manufacturing runs on paper. Not because companies are old-fashioned. Because regulations require it.
GMP compliance means paper inventory cards are the legal system of record. Every lot received, every status change, every consumption. All documented on physical cards that auditors can trace. This isn't bureaucracy. It's how you prove your products are safe.
But paper creates a visibility problem.
Planning teams can't see real-time inventory without walking the floor and manually reconciling cards. Material constraints get discovered when it's too late to adjust. QA spends hours tracking lot status across stacks of paper. Everyone knows there's a better way, but "go digital" isn't simple when paper IS the compliance mechanism.
The false choice everyone accepts
The industry assumes you have two options:
Option one: Replace paper with a validated digital system. This means a full ERP implementation or custom LIMS, 21 CFR Part 11 validation, change control across every process, regulatory filings, and a multi-year timeline. For a mid-sized pharma manufacturer, you're looking at millions of dollars and years of disruption, with real risk that auditors push back on the transition.
Option two: Stay paper-based. Accept that planning will always be reactive. Accept that inventory visibility requires manual reconciliation. Accept that your team spends 80% of their time keeping things running instead of improving them.
Both options are bad. There's a third way nobody talks about.
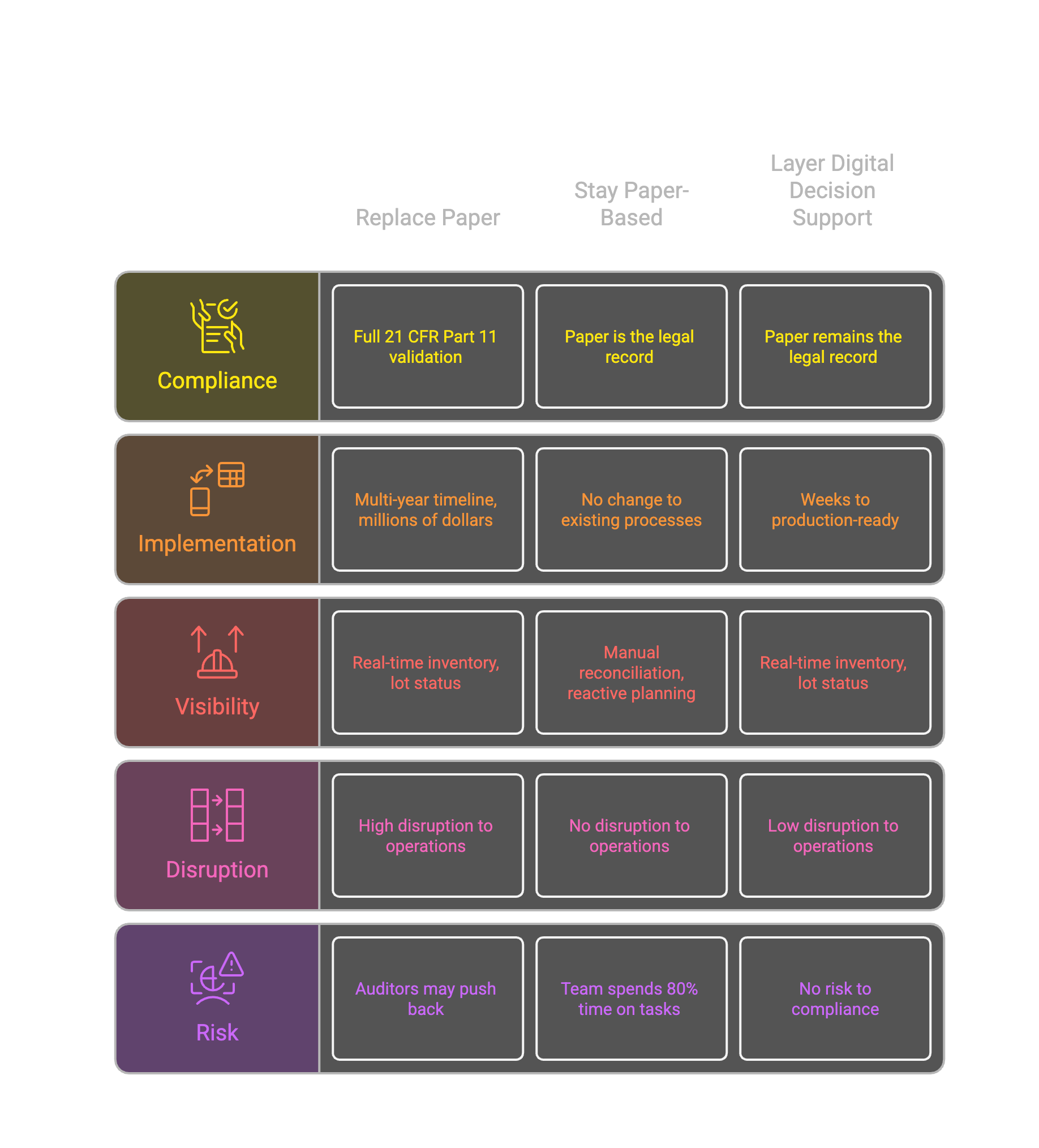
Layer, don't replace.
What if you could get digital visibility WITHOUT replacing your paper records?
The approach is straightforward: keep paper as the GMP system of record. It's what auditors expect. It's what your SOPs are built around. It's what works.
But layer a decision-support system on top. Use OCR to digitize inventory cards. Not to replace them, but to make them visible. The digital layer doesn't claim to be the legal record. It's a planning tool that happens to read from the real records.
Paper stays authoritative. Digital adds visibility.
This isn't a workaround. It's the right architecture. Your compliance posture doesn't change. Your paper records remain the source of truth. You just stop being blind to what's in them.
What we built for i3 Pharmaceuticals
We recently built this system for i3 Pharmaceuticals, a specialty pharmaceutical manufacturer. The project went from kickoff to production-ready in about ten weeks.
OCR Digitization
Inventory cards get scanned at receiving. OCR extracts material identification, receipt details, QA dates, and transaction rows. The original scans are retained as reference. You can always see exactly what the paper said. Verification workflows let users correct OCR errors with full audit trail of what was changed and why.
Demand Planning Matrix
The core planning interface is a matrix: raw materials on rows, batch products on columns. Planners input how many batches they're planning for each product. The system calculates total material requirements and compares against available inventory.
Visual indicators show status at a glance: green means sufficient and released, yellow means risk (retest pending or nearing expiry), red means shortage or quality hold. For the first time, planning teams can see constraints before they become problems.
Lot-Level Lifecycle Tracking
Every raw material lot is tracked: quantity received, quantity consumed, available quantity. Quality status flows through the expected lifecycle (quarantined, released, on hold, rejected, conditional release) with expiry and retest dates visible throughout.
Two-person verification for critical actions. System-derived expiry status. Complete visibility into material constraints without touching the paper workflow.
21 CFR Part 11 Compliance
Electronic signatures for status changes. Complete audit trail capturing user, timestamp, previous value, new value, and reason for every change. Role-based access control. Data integrity controls that prevent deletion of GMP-relevant records.
The digital layer is compliant. It just doesn't claim to replace the paper.
Why auditors won't push back
The key insight is what we're NOT doing. We're not replacing GMP controls. We're not asking auditors to accept a new system of record. We're not changing SOPs or retraining the floor on new compliance workflows.
We're adding a decision-support layer that:
Gives planning teams visibility they never had. Lets QA track status without manual reconciliation. Enables proactive identification of material constraints. Maintains complete compliance with existing GMP requirements.
Paper stays the legal record. The digital system just makes it visible.
Get the visibility. Keep the compliance.
"Replace everything" projects take years and often fail. Staying paper-based means staying blind.
Layer digital decision support on top of existing GMP controls. Ship in weeks, not years.